Panel-based and hotspot testing may miss genomic variants—some of which may be actionable1,2
| DNA | RNA | Paired Tumor-Normal Match | |
|---|---|---|---|
| Hotspot DNA | ✓ | ||
| Hotspot RNA | ✓ | ||
| Comprehensive genomic profiling (Fixed panel DNA sequencing) |
✓ | ||
| OncoExTra® (whole-exome DNA sequencing + whole-transcriptome* RNA sequencing) |
✓ | ✓ | ✓ |
Allows for comprehensive analysis of all protein-coding genes in a sample
Allows the identification of transcript variants and fusions that may be undetectable through conventional CGP tests, which only employ DNA analysis3
*Whole-transcriptome with select variants reported in New York State

Understand the impact the test has on patients
Analytic validation and clinical utilization of the comprehensive genomic profiling test, GEM ExTra®.‡
‡ OncoExtra was previously known as GEM ExTra.
The value of comprehensive genomic sequencing to maximize the identification of clinically actionable alterations in advanced cancer patients: a case series.
GEM ExTra, OncoExTra; TMB, tumor mutational burden.
Detect more variants:
The OncoExTra test delivers the complete genomic story
DNA
Thoroughly interrogates~20,000 genes through WES3
RNA
Sequences all transcriptspresent through WTS
Utilize patient-matched tumor-normal sequencing to further refine therapy selection
- Limit false positives by identifying and ruling out benign variants in a patient’s tumor sample vs normal blood sample3
- Reduce the overestimation of tumor mutational burden (TMB) calculations by excluding mutations also found in the normal sample3,4
Proven to reveal more clinically actionable information3
Actionable variants were found across many different types of solid tumors3

98% (164/167)
Colorectal Cancer3

96% (53/55)
Lung Cancer3

96% (25/26)
Bladder Cancer3

93% (80/86)
Breast Cancer3
A clinical study found that out of 75 fusions detected in
DNA+RNA by OncoExTra, 31 were found only in RNA3 *
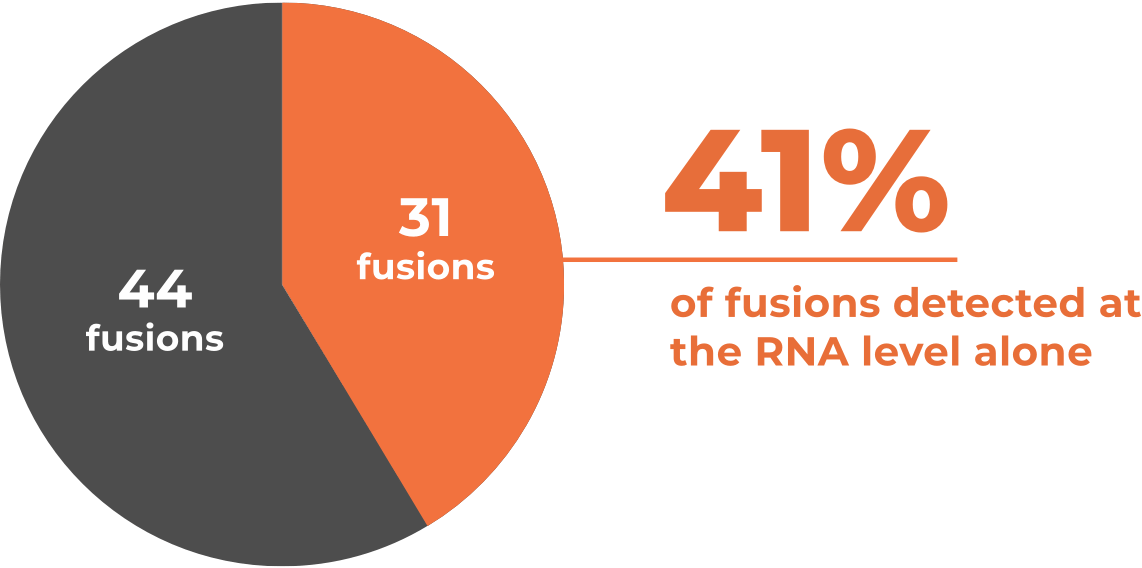
*Retrospective analysis of 1509 clinical reports, of which 1261 included both DNA and RNA profiling. Among the 75 reports with clinically actionable fusions detected in the RNA, 31 had RNA findings only.3
†Clinically actionable variants are defined as variants that are associated with available therapies or clinical trial enrollment for a specific somatic variant identified in a patient’s tumor.3
-
References


